Triplex Respiratory Pathogen Panel: HVRe Influenza, RSV, SARS-CoV-2 Detection
Empower your diagnostics with our Triplex Respiratory Pathogen Panel, offering precise molecular detection for HVRe Influenza, RSV (Respiratory Syncytial Virus), and SARS-CoV-2. Unrivaled accuracy in identifying key respiratory viruses, providing clinicians with essential insights for targeted and effective patient care strategies. Stay ahead in respiratory health with advanced molecular testing.

Advanced Respiratory Pathogen Detection: CE-IVD Approved Point-of-Care PCR
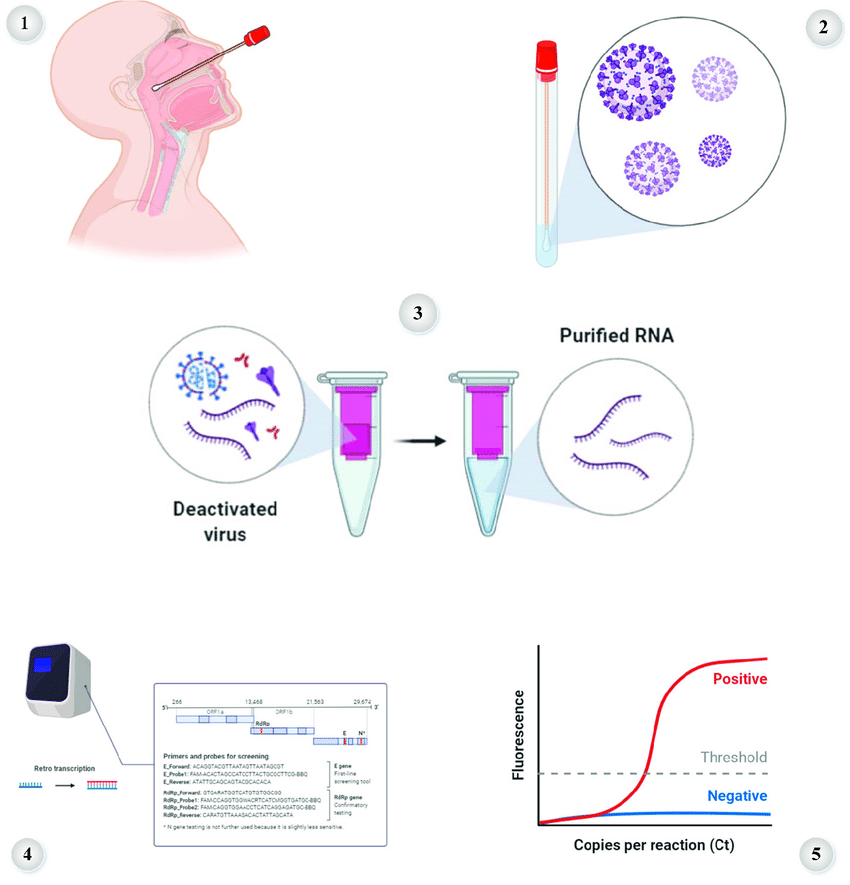
Enhance your diagnostic capabilities to unprecedented levels with our cutting-edge Respiratory Pathogen Detection solution, proudly bearing the CE-IVD approval, a testament to its compliance with stringent regulatory standards. Meticulously crafted, this solution is adept at reliably identifying and distinguishing the most prevalent respiratory pathogens of the season. Revolutionize your diagnostic approach by conducting precise point-of-care PCR testing right at the patient's bedside, empowering healthcare professionals to make immediate, informed decisions regarding diagnosis and treatment.
Powered by state-of-the-art Sensitive Real-Time-PCR technology, our solution ensures rapid results delivery, with a remarkable turnaround time of just 75 minutes. This not only expedites the diagnostic process but also streamlines patient care, fostering timely interventions. The user-friendly sample-to-answer workflow has been designed for simplicity, catering to a diverse range of users and skill levels. Moreover, our solution's room-temperature stability not only facilitates cost-effective shipping but also ensures optimal storage conditions, further maximizing efficiency.
Whether deployed in a clinical laboratory or various care settings, our CE-IVD approved products seamlessly integrate confirmatory testing into existing workflows. This is particularly valuable in markets that recognize the CE marking as a gold standard for regulatory approval, underscoring our commitment to delivering high-quality solutions. For non-diagnostic research procedures, our Research Use Only (RUO) products offer tailored options, demonstrating our versatility in catering to diverse laboratory needs. Elevate your diagnostic standards with our comprehensive solution, combining accuracy, speed, and user-friendly design for unparalleled performance in respiratory pathogen detection.
HVRe Influenza / RSV / SARS-CoV-2 Respiratory Panel:
Fast, Accurate Diagnosis at the Point-of-Care
In today's fast-paced healthcare environment, rapid and accurate diagnosis of respiratory infections is crucial for effective patient management and preventing the spread of disease. The HVRe Influenza / RSV / SARS-CoV-2 Respiratory Panel is a revolutionary diagnostic tool designed to meet these critical needs.
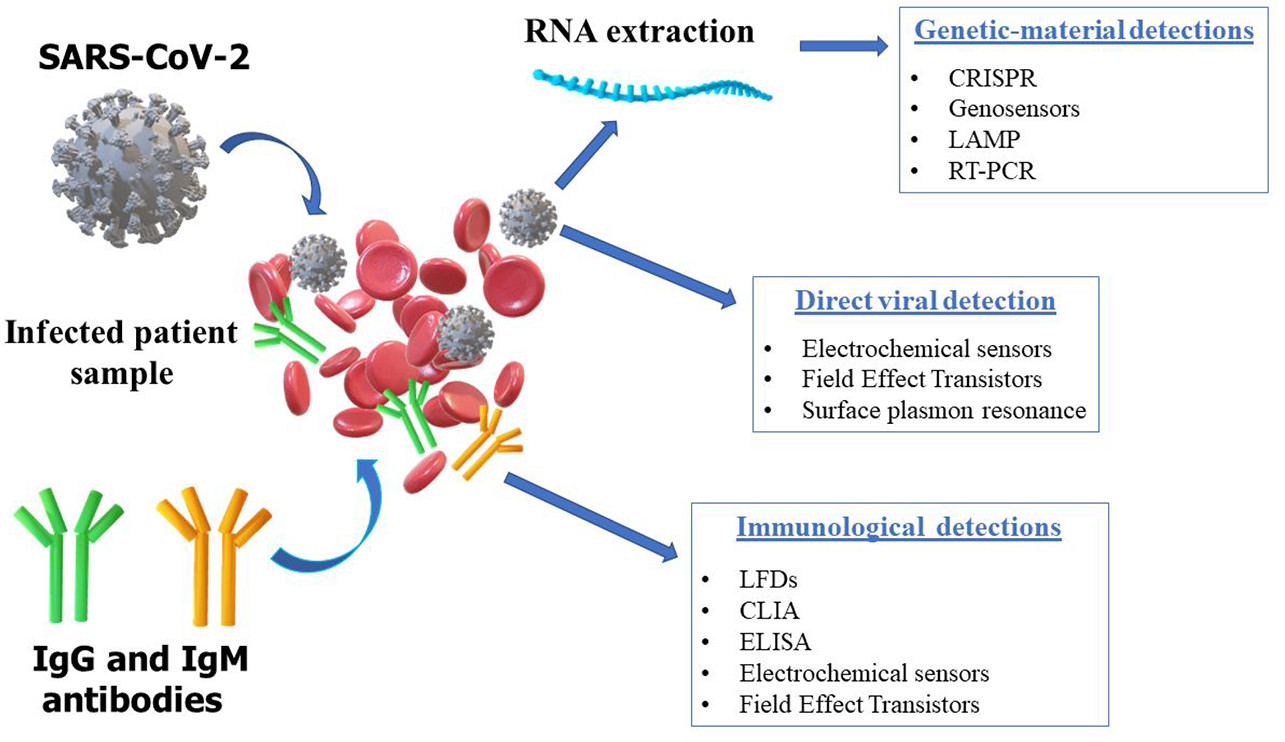
Unparalleled Benefits:

- Multiplex Detection: Simultaneously detects influenza A and B viruses, respiratory syncytial virus (RSV), and SARS-CoV-2 in a single test.
- Rapid Results: Provides accurate results in as little as 75 minutes, allowing for immediate treatment decisions.
- Point-of-Care Testing: Enables testing in diverse healthcare settings, including clinics, urgent care centers, and even physician offices.
- Simple Workflow: User-friendly design minimizes training requirements and streamlines the testing process.
- Reliable Performance: High sensitivity and specificity ensure confident results you can trust.
- Cost-Effective Solution: Room-temperature stability eliminates the need for expensive cold chain storage and reduces transportation costs.
Enhanced Patient Care:
- Improved Patient Outcomes: Early and accurate diagnoses empower healthcare providers to initiate appropriate treatment quickly, leading to better patient outcomes and reduced complications.
- Reduced Hospitalization Rates: Timely diagnoses can help prevent unnecessary hospital admissions, lowering healthcare costs and improving resource allocation.
- Enhanced Infection Control: Rapid identification of infected individuals allows for immediate isolation and containment measures, minimizing the spread of contagious respiratory illnesses.
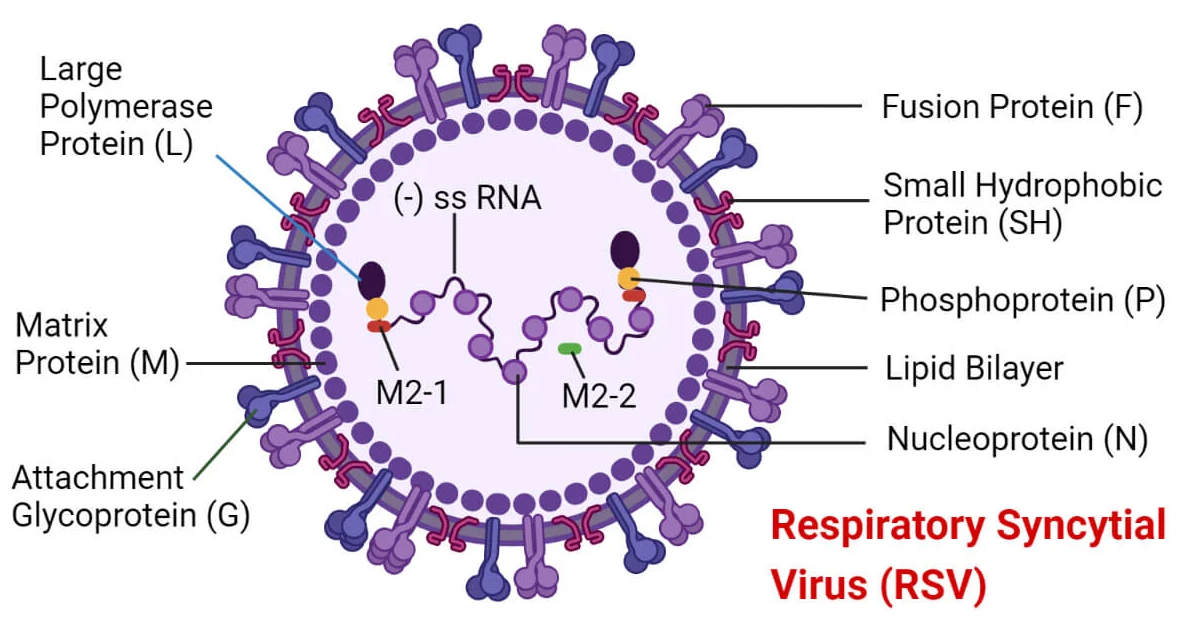
The HVRe Influenza / RSV / SARS-CoV-2 Respiratory Panel is the ideal solution for healthcare providers seeking:
- Efficient and accurate diagnosis of multiple respiratory infections
- Improved patient outcomes and reduced healthcare costs
- Enhanced infection control and public health protection
Beyond the Diagnosis: Empowering Action with the HVRe Respiratory Panel

The HVRe Influenza / RSV / SARS-CoV-2 Respiratory Panel goes beyond mere diagnosis. It equips healthcare professionals with the knowledge and tools necessary to take decisive action and optimize patient care.
Here's how the HVRe panel empowers action:
1. Tailored Treatment Plans:
By identifying the specific virus causing the patient's symptoms, the HVRe panel allows for targeted treatment. This can include:
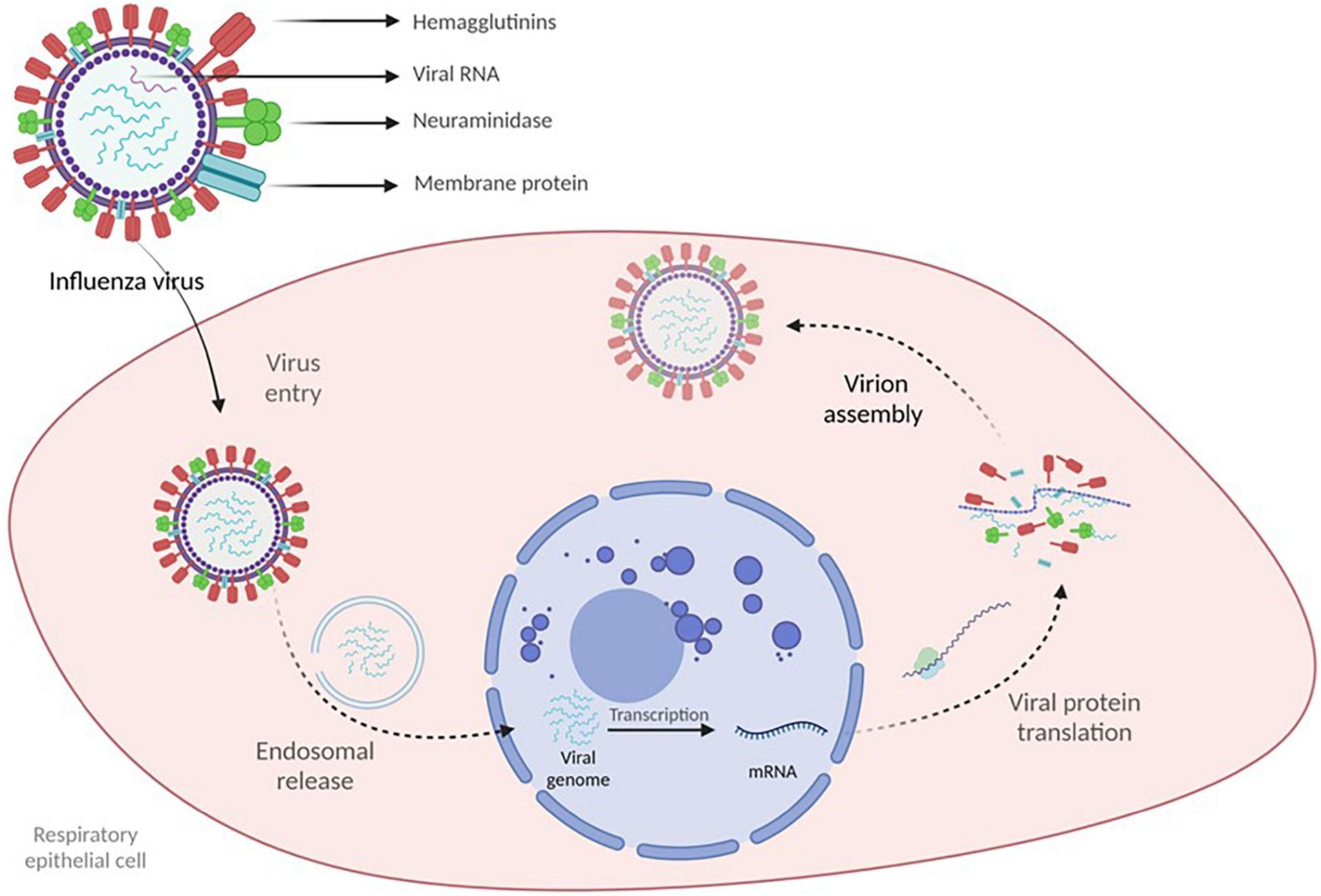
- Antiviral medications for influenza and RSV: These medications can significantly shorten the duration of illness and reduce the risk of complications.
- Antibiotics for bacterial co-infections: Accurate diagnosis helps differentiate viral from bacterial infections, preventing unnecessary antibiotic use.
- Supportive care: Based on the specific virus identified, healthcare providers can offer personalized recommendations for symptom management and supportive care.
2. Enhanced Infection Control:
Rapid identification of highly contagious viruses like SARS-CoV-2 enables prompt implementation of infection control measures. These measures may include:
- Isolation of infected individuals: This helps prevent the virus from spreading to other patients and healthcare workers.
- Enhanced hygiene and sanitation practices: Implementing strict hand hygiene protocols and disinfecting surfaces frequently minimizes the risk of further transmission.
- Contact tracing: Identifying individuals who have been in close contact with an infected person allows for preventive measures and early detection of new cases.
3. Informed Public Health Decisions:
By providing accurate and timely data on the prevalence of different respiratory viruses, the HVRe panel supports informed public health decisions. This information can be used to:
- Target vaccination campaigns: Prioritizing vaccination for populations at high risk for specific viruses based on real-time data.
- Allocate resources effectively: Directing resources towards areas with the highest burden of disease.
- Develop and implement effective public health interventions: Tailoring interventions based on the specific viruses circulating in the community.
4. Improved Patient Engagement:
Understanding the specific infection empowers patients to take an active role in their recovery. This includes:
- Adherence to treatment plans: Knowing their specific diagnosis encourages patients to follow treatment recommendations diligently.
- Symptom management: Understanding the expected course of illness based on the identified virus allows patients to manage symptoms effectively.
- Preventive measures: Patients can take individual steps to prevent the spread of the virus to others and protect their own health.
The HVRe Respiratory Panel empowers action at every level, from individual patient care to informed public health decisions. By providing accurate and timely diagnoses, it facilitates optimal treatment, enhances infection control, and fosters a collaborative approach to managing respiratory illnesses.
Conclusion:
Elevating Respiratory Diagnostics through Innovation and Precision
In the ever-evolving landscape of respiratory health diagnostics, the HVRe Influenza / RSV / SARS-CoV-2 Respiratory Panel stands as a beacon of innovation and precision. With its advanced molecular capabilities, this panel redefines the standards for pathogen detection, offering a comprehensive solution that goes beyond mere identification.
Our commitment to reliability is showcased through the panel's adeptness in differentiating between the most prevalent respiratory pathogens of the season. The CE-IVD approval underlines our dedication to meeting stringent quality and regulatory standards, ensuring clinicians can confidently rely on the accuracy of our diagnostic insights.
The integration of point-of-care PCR capabilities further cements our commitment to streamlined patient care. Diagnosis and treatment become seamless in the same patient visit, facilitated by the Real-Time-PCR technology that delivers sensitive results within an impressive 75-minute timeframe.
Ease of use remains at the forefront of our design philosophy, with a user-friendly sample-to-answer workflow that accommodates a diverse range of healthcare professionals. This adaptability extends to various care settings, providing flexibility for clinicians to deploy confirmatory testing where it matters most.
Optimal storage and shipping efficiency, coupled with room-temperature stability, not only enhance the longevity of samples but also contribute to the overall efficiency of diagnostic processes. Logistical complexities are minimized, ensuring that the focus remains on delivering timely and accurate results.
Global regulatory compliance is a cornerstone of our commitment to excellence. With CE-IVD products meeting the standards of markets recognizing CE marking, our panel assures clinicians of its reliability and adherence to rigorous regulatory requirements. For research purposes, our Research Use Only (RUO) products offer a specialized avenue for non-diagnostic research procedures.
In conclusion, the HVRe Influenza / RSV / SARS-CoV-2 Respiratory Panel represents more than a diagnostic tool; it embodies a paradigm shift in respiratory health diagnostics. By combining innovation, precision, and a global commitment to excellence, this panel empowers healthcare professionals with the insights they need to navigate the complexities of respiratory care, ultimately advancing patient outcomes and contributing to a new era of diagnostic excellence.